Description
Ag/AgCl reference electrode is used in a neutral environment. It consists of a Teflon cap with an AgCl-coated Ag wire, glass tubing, and a porous Teflon tip sealed into the glass tubing.
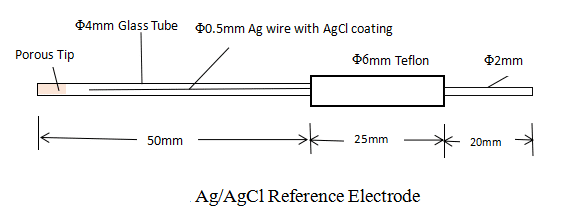
The porous Teflon tip has the following advantages: slow solution leakage and good durability due to its perfect chemical stability. The electrode should always be kept dipped in KCl solutions when it is not in use. If it is placed in the air by accident, the solution inside the porous structure will dry out which causes high resistance.
The reference electrode compartment (the glass tubing) is filled with 3.5M KCl aqueous solution. The potential of the reference electrode versus the Standard Hydrogen Electrode (0.0V) can be calculated from the activity (not concentration) of the Cl ion.
The solution in the compartment is refillable.
Maintenance
- The Ag/AgCl reference electrode (Silver/Silver chloride reference electrode) should be dipped in a 3.5M KCl solution after use.
- For Ag/AgCl reference electrode, when the liquid inside the tube is dry, 3.5M KCl solution should be refilled.
- Ag/Agcl reference electrodes are used at 0-60 degrees centigrade.
Comparison of Ag/AgCl Reference Electrodes
| Product | Specifications | Electrode Picture |
|---|---|---|
| Silver-Silver Chloride (Ag/AgCl) Electrode R0302 | 4mm (Diameter of glass rod) (3.5M KCl) |  |
| Silver-Silver Chloride (Ag/AgCl) Electrode R0303 | 6mm (Diameter of glass rod) (3.5M KCl) |  |
| Silver-Silver Chloride (Ag/AgCl) Electrode R0305 | Saturated KCl | 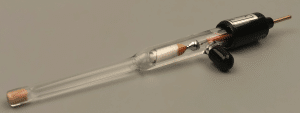 |


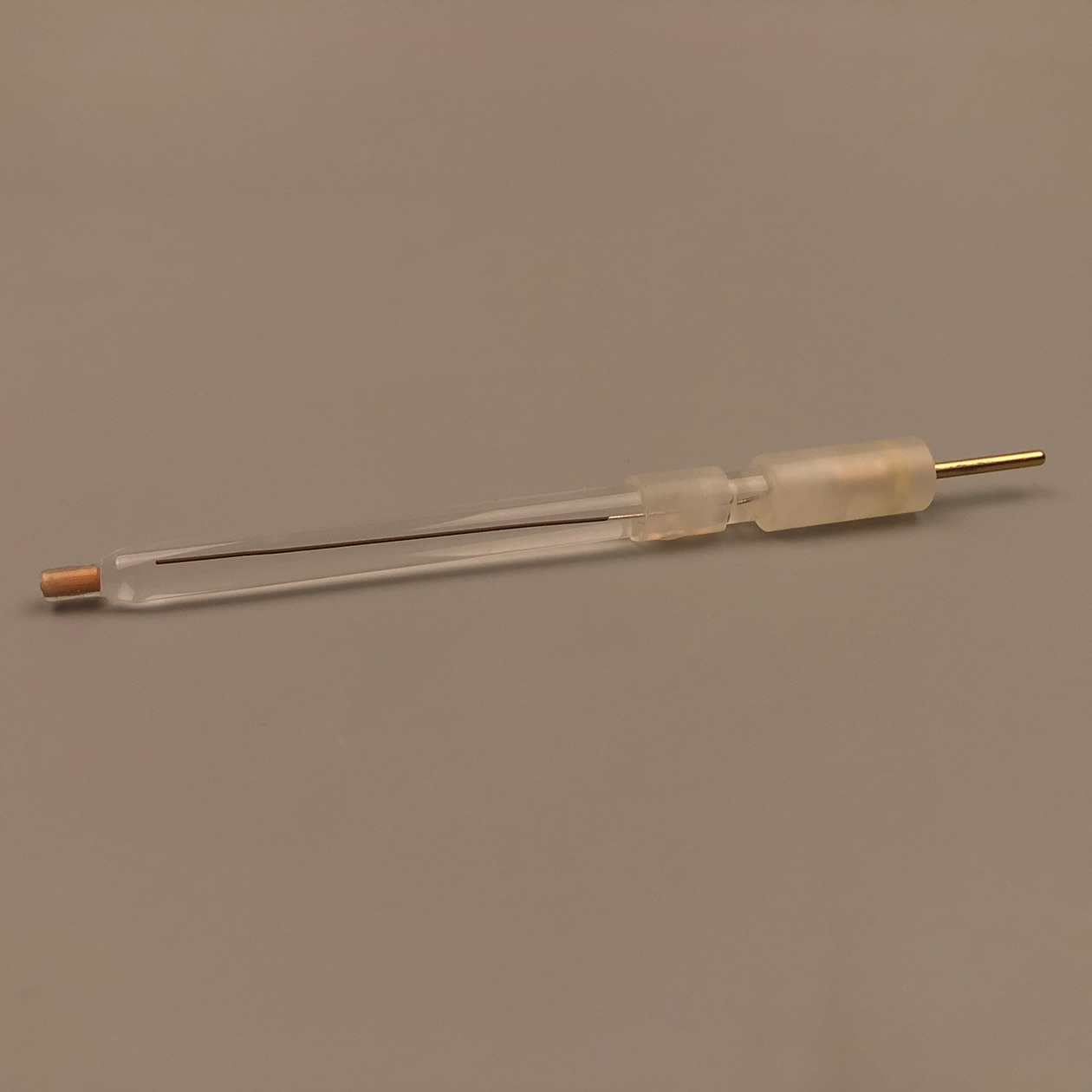



Reviews
There are no reviews yet.